Explain the fda test and query abroad
Recently, the U.S. Food and Drug Administration (fda) issued a statement on its official website, emphasizing that fda has never issued a so-called "registration certificate" to any medical device company or institution, that is, the "FDA registration certificate" on the market. .
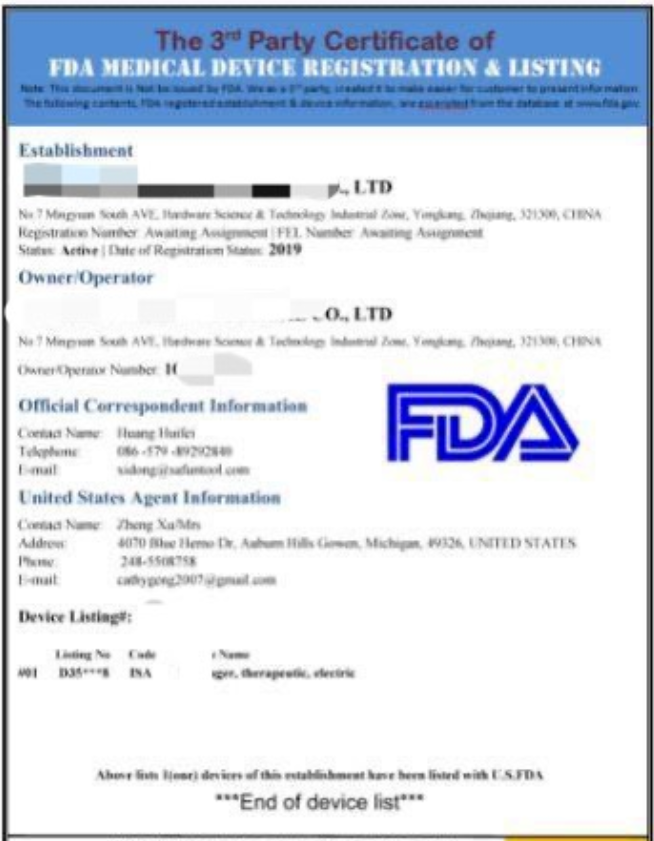
This official announcement mainly clarified the following points. No confirmation certificate will be issued for products or companies that have been registered or listed. The company registration and product listing information does not mean that FDA has approved the company and its products. FDA will not issue registration certificates to medical device companies.
This announcement is undoubtedly a bolt from the blue for some export companies that have been deceived by irregular certification agencies and are keen to spend money to apply for FDA registration certification.
The US FDA 510(k) "certification" of medical devices is based on different risk levels. The FDA test divides medical devices into three categories (I, II, and III), with Category III having the highest risk level. FDA clearly stipulates product classification and management requirements for each medical device. At present, there are more than 1,700 FDA medical device product catalogs. For any kind of medical device to enter the US market, it must first clarify the classification and management requirements of the product applied for listing.
After clarifying the above information, the company can begin to prepare the relevant application materials, and declare to the fda according to certain procedures to obtain approval. For any product, companies need to register and list products.
For Class I products (about 47%), general control is implemented. Most products only need to be registered, listed and implemented GMP regulations, and the products can enter the US market (a few of them are exempt from GMP, and a very small number To keep the product, you need to submit a 510 (K) application to the FDA (PMN (Premarket Notification));
For Class II products (approximately 46%), special controls are implemented. After registration and listing, companies still need to implement GMP and submit 510(K) applications (very few products are 510(K) exemptions);
For Class III products (accounting for about 7%), pre-market approval is implemented. After registration and listing, companies must implement GMP and submit a PMA (Premarket Application) application to the fda (some Class III products are still PMN).
For Class I products, after the company submits relevant information to the FDA, the fda query will only make an announcement, and no relevant certificates will be issued to the company. For Class II and III devices, the company must submit a PMN or PMA. The formal market access approval letter allows companies to directly sell their products on the US medical device market in their own name.
As for whether to go to the company for on-site GMP assessment during the application process, it is determined by fda based on comprehensive factors such as product risk levels, management requirements and market feedback.
Based on the above content, it can be seen that most products can obtain fda certification after enterprise registration, product listing and implementation of medical device GMP, or submit a 510 (K) application.

