Lihang teaches you how to distinguish the authenticity of EU ce certification
In 2020, everyone's lives have been greatly affected by the epidemic, and Europe has become a severely affected area due to the rapid spread of the epidemic. In order to better prevent and control the epidemic, resources such as medical masks, protective clothing and other medical supplies have become scarce products in the European market, so Europe has a great demand for protective supplies. For products to be exported to the EU market, the EU CE certification is a must.
There are many types of medical CE certificates on the market today. Today we will talk about how to identify them so that everyone can know how to check the authenticity of CE certificates.
The first way is to check the official website of the notified body. In some large organizations that have obtained EU certification and authorization, they will open a window for querying certificates on their official websites. After logging into the official websites of these institutions, we can enter the manufacturer’s English name, certificate number and other information in the query window to check whether there is a matchCE certificationThe certificate appears. If it appears, it means that the certificate is true, otherwise it is false. This method still requires our own judgment. If the official website of the institution we found does not open the certificate query service, then this method is not applicable. In response to this situation, when we get a medical CE certificate, how do we identify whether it is issued by an EU authority certification body?
The second way is to start with the certificate issuing agency we have, and go to the official website of EU to check to see if it has the EU Medical Device Directive MDD 93/42/EEC or MDR Medical Device Regulation (EU) 2017/745 The corresponding certification qualifications.
The official website of the European Union MDD 93/42/EEC Medical Devices Directive authorized institutions to query the address, you can contact Lihang to send it to you.
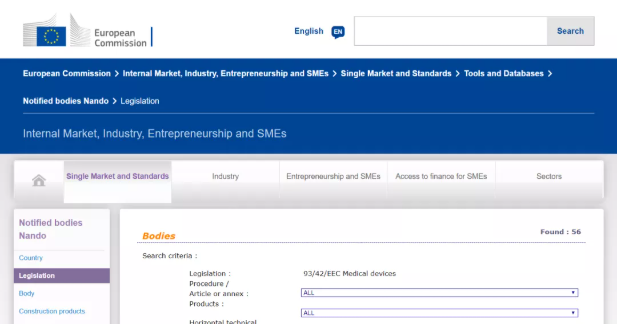
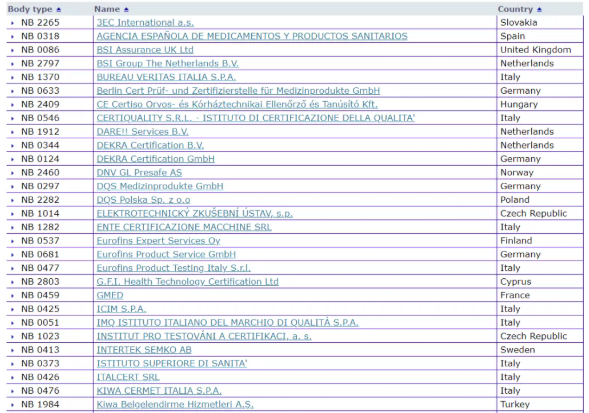
It can be seen from the EU official website that there are 56 notified bodies authorized by the MDD 93/42/EEC Medical Device Directive. The specific organization list, bulletin number, and the scope of their qualified products are listed in detail.
From May 26, 2020, the MDR (EU) 2017/745 Medical Device Regulation will formally replace the mandatory implementation of the EU’s current MDD Medical Device Directive, which can also be found on the EU’s official website. There are currently only 12 notified bodies with MDR authorization .
The official website of the European Union MDR (EU) 2017/745 medical device regulations authorized institutions to query the address, you can contact Lihang to send you.
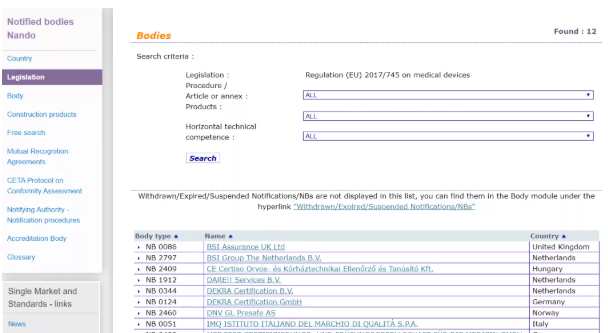
Therefore, if your medical EU CE certificate issuance agency is not in the above list, it means that it does not have the EU certification qualification for medical products, let alone the issuance of CE certificates. Then, I regret to say , The "CE certificate" you got is invalid.
Previous: Explain the fda test and query abroad
下一条: No Information

